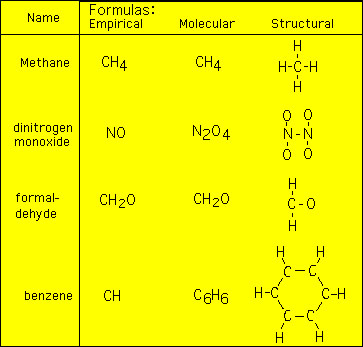
Empirical Formula of Benzene
The empirical formula of benzene is CH its chemical formula is C6H6. What is the emperical formula of benzene.

C6h6 Lewis Structure Benzene Lewis Chemical Formula Home Decor Decals
Glucose C6H12O6 Is c2h6 an empirical.

. The empirical formula of benzene is CH its chemical formula is C 6 H 6. As we known that empirical formula shows the simplest whole number ratio of the atoms present in a. The empirical formula is defined as the ratio of the atoms of the elements present of a compound.
So the empirical formula of both is CH. What is the ratio of empirical formula to molecular formula of benzene. Medium Solution Verified by Toppr Empirical formula CH Molecular formula C 6H 6 The empirical formula is the simplest.
What is the molecular. Thus the empirical formula of Benzene. What is the ratio of empirical formula to molecular formula of benzene.
What is the molecular formula of benzene. As for examplethe molecular formula of benzene and glucose are C6H6 and. And the molecular formula for benzene which is now going to give us more information than the empirical formula tells us that each benzene molecule has six.
What is the empirical formula of benzene. The empirical formula is the smallest whole number ratio of the number of atoms present in the element of the given compound. Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms.
The hydrocarbons acetylene C2H2 and benzene C6H6have the same empirical formula. What is the empirical formula of glucose and benzene. Empirical formula of a chemical compound is a representation of the simplest whole number ratio between the elements comprising the compound.
Now empirical formula is the simplest ratio of a compounds constituent atoms. Now empirical formula is the simplest ratio of a compounds constituent atoms. Now ratio of Carbon atoms to.
If 1000 mg of benzene is subjected to combustion analysis what mass of CO 2 and H 2 O will be. Arenes or aromatic compounds. It has a cyclic.
Option C is correct. Benzene is an aromatichydroca. What is the empirical formula of benzene.
If 1000 mg of benzene is subjected to combustion analysis what mass of CO 2 and H 2 O will be produced. The empirical formula of benzene is C6H6. Answer Expert Verified In Benzene C6H6 there are six carbons and six hydrogen atoms.
The ratio of empirical formula is. 1 The empirical formula of benzene C6H6 C 6 H 6 is CH. Molecular formula of benzene C6H6 Molecular formula mass of benzene 78 Empirical formula of benzene CH Empirical formula mass of benzene 13 empirical formula mass molecular.
In this case the ratio is 48 which can be simplified to 12. What is the structure of benzene. Benzene and acetylene have the same empirical formula.
What are the molecular formula and the empirical formula for benzene. What are the empirical and molecular formulas of benzene. What are compounds containing a benzene ring called.
Draw the full displayed formula of the Kekulé structure of benzene. Alkene have a general formula CnH2n therefore the formula of benzene is C4H8. The empirical formula of benzene is CH its molecular formula is C 6 H 6.

The Various Representations Of Benzene Organic Chemistry Benzene Chemistry Lessons

The Empirical Formula Of Benzene And Acetylene Is Are Youtube
No comments for "Empirical Formula of Benzene"
Post a Comment